Have you ever felt unreasonably tired all the time? Have you experienced apathy, drowsiness, and general weakness for no apparent reason, despite having enough sleep? If so, it is worth examining the level of elements in the body, because iron deficiency may be the cause.
Have you ever felt unreasonably tired all the time? Have you experienced apathy, drowsiness, and general weakness for no apparent reason, despite having enough sleep? If so, it is worth examining the level of elements in the body, because iron deficiency may be the cause.
A MICROELEMENT WITH A MACRO SIGNIFICANCE
The human body contains about 60 elements essential for its proper functioning. They can be divided into macroelements, the content of which in the body is over 0.01% and the demand for which exceeds 100 mg per day, and microelements with the daily requirement below 100 mg and the level in the body lower than 0.01%. Although iron is an element called belonging to the second group, its deficiency can lead to serious physiological disorders. Iron is an essential element for the body. Without it, cellular respiration, one of the most commonly used indicators of life processes, would not be possible.
The human body obtains over 80% of iron from the breakdown of red blood cells (hemoglobin), the rest from stored supplies and food intake. In the body of an adult weighing 70 kg, there is an average of 4-5 g of iron, of which 60-70% is found in red blood cells, 11-14% in myoglobin (muscle pigment), and about 3% in oxidative enzymes conditioning the process cellular respiration and catalase enzymes that break down toxic hydrogen peroxide. The remaining amount of iron is stored in the liver, spleen, kidneys and bone marrow as ferritin (in combination with protein) and hemosiderin (in complex combination with proteins, carbohydrates and lipids).
Iron is removed from the body in a small amount with feces, urine and sweat, as well as with the exfoliation of the epidermis. In disease situations related to bleeding in the gastrointestinal tract (e.g. in chronic inflammation of the intestinal mucosa, irritable bowel syndrome and celiac disease, food intolerances, various types of allergies or peptic ulcer disease), in the case of constant intake of acetylsalicylic acid, and due to heavy menstruation in some women, iron losses can be much greater. Athletes are also at increased risk of iron deficiency. However, congenital dysfunctions of iron utilization by the body are rare.
DEMAND
The demand for iron varies depending on the age, gender and health of the body, and the level of its satisfaction depends largely on the diet. There are differences in the absorption of iron from different types of food. From 1-2% for cereal products, up to 25% for a meat diet and about 10% for a mixed diet. This means that people on a mixed diet should consume about ten times more iron than the body needs.
ROLE FOR THE BODY AND EFFECTS OF DEFICIENCY
Iron, as a component of hemoglobin, which transports oxygen from the lungs to individual cells of the body, and myoglobin, is essential in the breathing process. It binds carbon dioxide and passes it to the lungs, where it is removed from the body. It actively participates in in DNA synthesis, affects the proper functioning of the immune system. It also affects the body’s energy capabilities, such as physical and mental fitness.
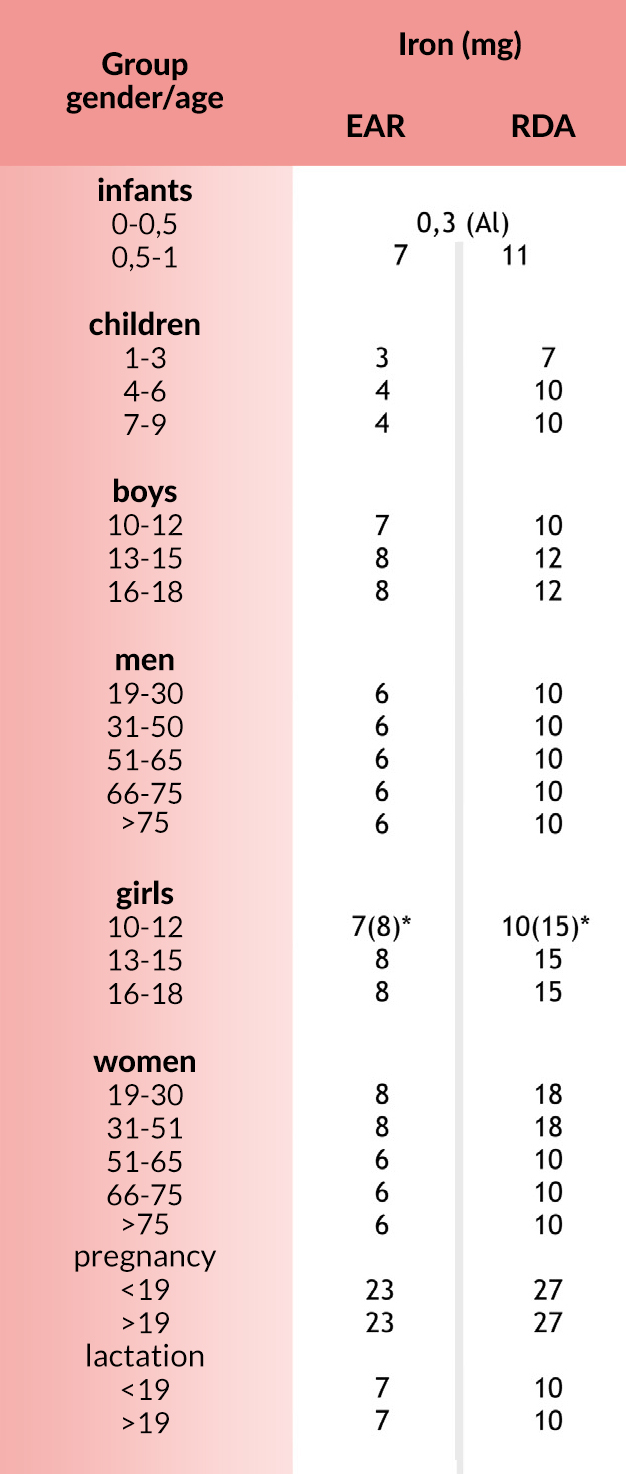
Norms
EAR (Estimated Average Requirement) average demand for the group during the day.
RDA (Recommended Dietary Allowances) recommended intake for individuals during the day.
AI (Adequate Intake) sufficient intake during the day. These standards have been found to be sufficient on the basis of experimental studies or observations of average food consumption and apply to individual and group nutrition.
Iron is an essential element for the body. Without it, cellular respiration would not be possible; this is one of the most used indicators of life processes.
In a situation where the supply of iron is lower than the body’s needs, and this condition is prolonged, the reserves accumulated in the liver and other internal organs are exhausted. This leads to illnesses such as hypochromic anemia, i.e. iron deficiency anemia.
Iron deficiency is dangerous for many reasons. Since it actively participates in the transport of oxygen to all cells, its lack significantly worsens the overall condition of the body. Too low a level of this element can cause brain hypoxia, which may result in impaired concentration, chronic fatigue, and even states of exhaustion, apathy or hyperactivity that are difficult to justify with another cause.
Iron deficiency can also be the cause of chronic pain and dizziness, ringing in the ears, scotoma or deterioration of memory processes. The lips and skin become pale, the nails become brittle, and the hair structure weakens, splits and begins to fall out. There may be disturbances in the work of the heart, the circulatory and respiratory capacity decreases. Symptoms of oxygen starvation in the body are visible, caused mainly by myocardial hypoxia, which in turn may lead to tachycardia, arrhythmias, systolic murmurs and stenocardial ailments, predisposing to myocardial infarction.
Research conducted in 2017 (Trotti LM, Becker LA) proved that low iron levels may be one of the factors causing restless legs syndrome. People with low iron levels may occasionally develop appetite alterations, including cravings for inedible or very cold and crunchy foods, or gastrointestinal dysphagia (Plummer-Vinson syndrome).
Iron deficiency affects general health, including sleep quality. It becomes impossible to sleep, despite the appropriate number of hours of rest. People with low blood iron levels often wake up feeling more tired than when they went to bed.
Iron is involved in the transport of oxygen to all cells, therefore, its lacks causes hypoxia, which in turn is the cause of concentration disorders, fatigue and exhaustion. People with low blood iron levels often wake up feeling more tired than when they went to bed.
Iron is important not only for adults. People most at risk of anemia include infants over 4 months of age, children, and adolescents. Adequate supply of iron affects, among other things, the proper growth and mental and physical development of a young organism. Its deficiency impairs the functioning of the teenager’s brain by impairing the formation of myelin – a substance that is a kind of insulation, connecting nerve cells with each other, and at the same time accelerating conduction in the brain. Iron, by affecting metabolic processes, enables individual organs to develop properly. This is very important at every stage of growth of a young organism. Iron deficiency in boys causes poor muscle mass growth and a decrease in blood volume. However, especially in girls who lose a large amount of iron during menstruation, a low supply of this element can cause chronic fatigue, lack of appetite, difficulty remembering and lowering immunity. Statistics conducted in Germany show that more than 16% of teenagers are insufficiently supplied with iron. The reason for this is the consumption of a large amount of highly processed products, including fast food, dairy products, or the reduction or complete elimination of meat from the diet.
Adequate iron supply is very important for children and adolescents because it affects their proper growth and mental and physical development. Deficiency of this element is very common in children with ADHD.
IRON AND DIET
In the treatment of diseases associated with iron deficiency, a proper diet is very important. In food products, there is heme iron, absorbed on average in 20%, and less absorbed by the body, non-heme iron, absorbed in only 5% (for comparison, iron from breast milk is absorbed in 50%).
That is why mother’s milk is so important in infant nutrition). Iron is better absorbed from products containing its heme form. The source of heme iron are products of animal origin: liver, heart, kidneys, meat and its products, fish, and poultry. Important for vegetarians and anyone who does not eat animal products, are sources of non-heme iron. These include whole grains and cereal products such as flour, coarse groats, whole meal bread, wheat bran and germ, rice bran, and egg yolks. Green vegetables also abound in non-heme iron: spinach, parsley, chives, sorrel, cabbage, brussels sprouts, and dill, as well as dried fruits and vegetables, hazelnuts, avocados, peppers, chard, almonds, nettles, licorice, wild rose, and cocoa and baker’s yeast.
Many other factors also affect the bioavailability of iron. Its absorption from food is increased by the presence of vitamin C, low pH of gastric juice, as well as the presence of amino acids: histidine and lysine. Factors that hinder the absorption of iron include phytic acids (contained, among others, in seeds and grains), phosphates (in, among others, in cold cuts, drinks), casein and whey proteins (in milk, its products and in eggs) and some elements, such as calcium, manganese, copper, zinc, cadmium and lead. The bioavailability of iron is also significantly affected by the health of the body, especially infections, diseases of the gastrointestinal tract and the amount of its body stock.
Contrary to popular belief, the most valuable source of dietary iron is not the proverbial spinach. The non-heme iron contained in it is absorbed from the gastrointestinal tract in an amount of less than 5% of the amount supplied. Much better absorbed, in 10-25%, is heme iron, present primarily in red meat and fish.
DIAGNOSTICS
When to test your iron level? Certainly, in the above-mentioned situations of deterioration of well-being and, of course, for regular prevention. It is also very important to control the level of this element in the developing bodies of children and adolescents, pregnant and lactating women, as well as in athletes and regular blood donors. One of the effective methods to determine the concentration of iron in the body is the EHA test, i.e., elemental hair analysis. This test accurately shows the content of iron in the body and the ratio of its amount to other elements in the examined person.
The EHA test therefore allows you to determine the actual level of iron absorption. It should be remembered that the degree of absorption of an element into the body is influenced by its quantitative relationships with other elements. These interdependencies have a decisive impact on health. Disturbances in the proportions of individual minerals may indicate diseases of individual systems or organs.Thanks to the elemental analysis of the hair, it is possible to obtain extensive knowledge about the state of health of the body and to find out the cause of the decline in its condition.
Often only a comparison of the results of the blood test and the elemental analysis of the hair gives a full picture of the patient’s health:
Morphology – shows high levels of iron for a long time.
Elemental hair analysis – shows iron deficiency.
Conclusions – the human body has a limited ability to excrete iron. With a high supply, there may be excessive accumulation (perhaps even of a toxic nature) in individual organs: the liver, heart, and pancreas. This is a very dangerous situation because iron, which is a strong oxidant, can cause diseases of these organs, including heart rhythm disorders, liver disease, susceptibility to viral and bacterial infections. A high level of iron in the blood with a slightly lower level in the hair therefore indicates that the excess iron is not being excreted properly from the body (otherwise its presence in the hair would be higher). Confirmation of this diagnosis may also be a low level of zinc and manganese, iron being its natural antagonist.
Normal serum iron content:
Women: 6.6-26 mmol/L (37-145 mg/dL)
Men: 10.6-28.3 mmol/L (50-158 mg/100dL)
The correct iron content in the EHA test:14-24ppm
We talk about excess iron in the body in extreme situations. However, when it occurs, it poses a great danger to the body. It causes haemochromatosis (usually congenital, less often acquired), consisting in the deposition of excess iron in the pancreas, liver, myocardium, adrenal cortex, and joints, which leads to damage to the tissues of these organs. The lethal dose for an adult is about 200 mg/kg of body weight. Iron (II) salts are toxic to humans in large amounts. Iron compounds (III and IV) are not absorbed by the body; therefore, they are not harmful.
Another example is excess calcium in the diet. Too much supply of this element reduces the intestinal absorption of zinc, which in turn will affect the excessive absorption of copper. Copper in a toxic form can lead to poisoning of the body. As a result of very similar symptoms, such poisoning is often identified with iron deficiency and deterioration of blood results. Therefore, test results should always be consulted with specialists. With a doctor, as well as with a dietitian who will determine the right diet, and if necessary, will also help choose the right supplementation for the body.

Elżbieta Rymar – Nutritionist, member of the Polish Society of Dietetics (PTD) and the Polish Society of Nutritional Sciences (PTNŻ). Author of the book entitled the anti-stress diet.
Bibliography:
1. Bańkowski E.: Biochemistry. A textbook for students of medical schools. ed. II.
2. Urban E. & Partner, 2009, p. 406.
3. Biggs M, McVicar J, Flowerdew B: The Great Book of Vegetables, Herbs and Fruits. Warsaw: Bellona Publishing House, 2007, pp. 174–175. ISBN 83-11-10578-2.
4. Ciborowska H., Rudnicka A.: Dietetyka, PZWL Warszawa 2012, p. 150.
5. Human physiology with elements of applied and clinical physiology edited by
6. Traczyk W. Z. and Trzebski A.; ed. III amended and supplemented, p.125.
7. Requirements of Vitamin A, Iron, Folate, and Vitamin B12. Report of a Joint FAO/WHO Expert Consultation. FAO, 1988, pp. 33–50. ISBN 978-92-5-102625-0.
8. Papp John F: Industrial Minerals & Rocks: Commodities, Markets, and Uses. ed. 7. SME, 2006. ISBN 978-0-87335-233-8.
9. Kim M. O’Connor, Douglas S. Paauw – 2016 – Medical Neurotherapeutics 2014; 11: 177–87. American Academy of Neurology.
10. Klaus W. Lange et al., The Role of Nutritional Supplements in the Treatment of ADHD: What the Evidence Says, Current Psychiatry Reports, 19 (8), 2017, DOI: 10.1007/s11920-017-0762-1.
11. Rutherford John Gettens: Painting Materials: A Short Encyclopaedia. Courier Dover Publications, 1966, pp. 105–106. ISBN 978-0-486-21597-6.
12. Sheftel AD, Mason AB, Ponka P. The long history of iron in the Universe and in health and disease. Biochim Biophys Acta 2012; 1820: 161-187.
13. Trzebiatowski W.: Inorganic chemistry. ed. VIII. Warsaw: PWN, 1978, pp. 566–567.